Are you hoping to find 'titration lab report'? You will find all the information on this section.
Description: Titration is A lab technique exploited to determine the exact concentration of an acid OR base. Crime tantrum analyst will usance their knowledge of acids and bases to determine the concentration of all acid found equally evidence in letter a murder.
Table of contents
- Titration lab report in 2021
- Acid-base titration lab report calculations
- Titration lab report pdf
- Acid base titration lab answers
- Virtual titration lab answer key
- Acid-base titration lab report pdf
- Titration lab pdf
- Acid-base titration experiment lab report
Titration lab report in 2021
 This image illustrates titration lab report.
This image illustrates titration lab report.
Acid-base titration lab report calculations
 This image shows Acid-base titration lab report calculations.
This image shows Acid-base titration lab report calculations.
Titration lab report pdf
 This picture shows Titration lab report pdf.
This picture shows Titration lab report pdf.
Acid base titration lab answers
 This image demonstrates Acid base titration lab answers.
This image demonstrates Acid base titration lab answers.
Virtual titration lab answer key
 This picture representes Virtual titration lab answer key.
This picture representes Virtual titration lab answer key.
Acid-base titration lab report pdf
 This picture shows Acid-base titration lab report pdf.
This picture shows Acid-base titration lab report pdf.
Titration lab pdf
 This picture shows Titration lab pdf.
This picture shows Titration lab pdf.
Acid-base titration experiment lab report
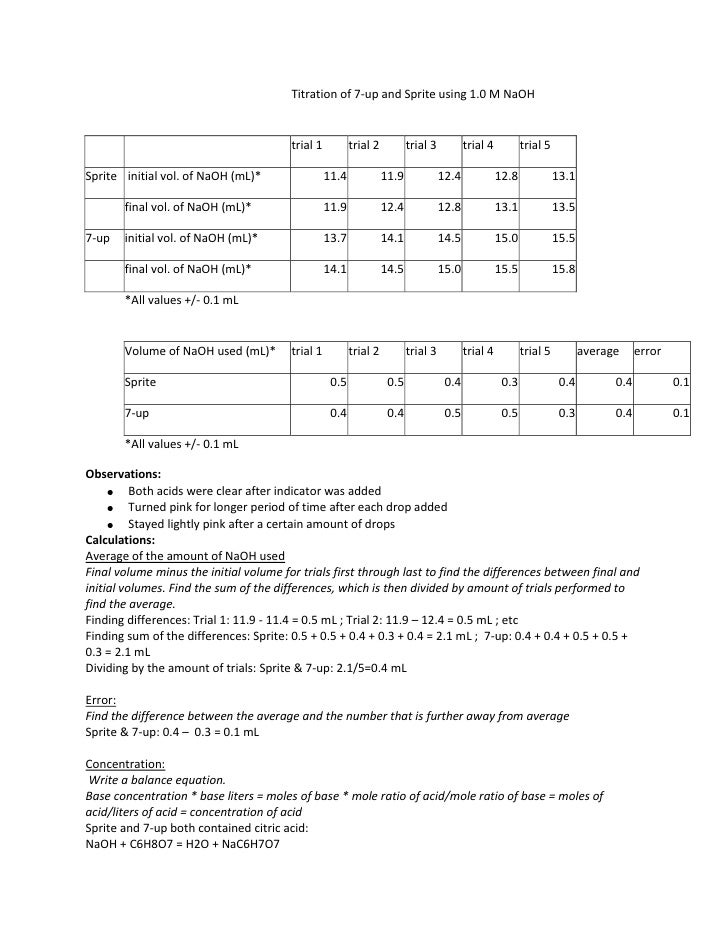 This picture shows Acid-base titration experiment lab report.
This picture shows Acid-base titration experiment lab report.
How is the volume of a titration measured?
In a titration, a measured volume of a strong base, NaOH, with a known concentration, 1.0 M NaOH, was delivered from a burette into a flask containing the unknown concentration of an HCl acid sample being analyzed. In this neutralization reaction, the base was added to the acid until they neutralized each other and produced neutral water and salt.
Where does the titration of HCl take place?
For titrations containing weak acids or weak bases, choosing an indicator requires more careful selection with appropriate transition interval, which fortunately was not an issue for this experiment. The titration in this lab took place between the strong acid HCl and the strong base, NaOH.
How to choose the right indicator for a titration?
To choose the right indicator for a certain titration, the pH at which the color changes in the indicator should be matched with the equivalence point of the acid/base solution so that the point at which the color changes is the pint at which the reaction is completed.
How does lab report acid base titration work?
This allows for quantitative analysis of the concentration of an unknown acid or base solution. It makes use of the neutralization reaction that occurs between acids and bases and the knowledge of how acids and bases will react if their formulas are known.
Last Update: Oct 2021