Do you scour the internet for 'thesis on electrocoagulation'? Here you can find questions and answers about the issue.
Table of contents
- Thesis on electrocoagulation in 2021
- Electrocoagulation reactor design
- Electrocoagulation review
- Design of electrocoagulation unit
- Electrocoagulation process
- Electrocoagulation wastewater treatment pdf
- Application of electrocoagulation
- Electrocoagulation in the treatment of industrial waters and wastewaters
Thesis on electrocoagulation in 2021
 This image representes thesis on electrocoagulation.
This image representes thesis on electrocoagulation.
Electrocoagulation reactor design
 This picture representes Electrocoagulation reactor design.
This picture representes Electrocoagulation reactor design.
Electrocoagulation review
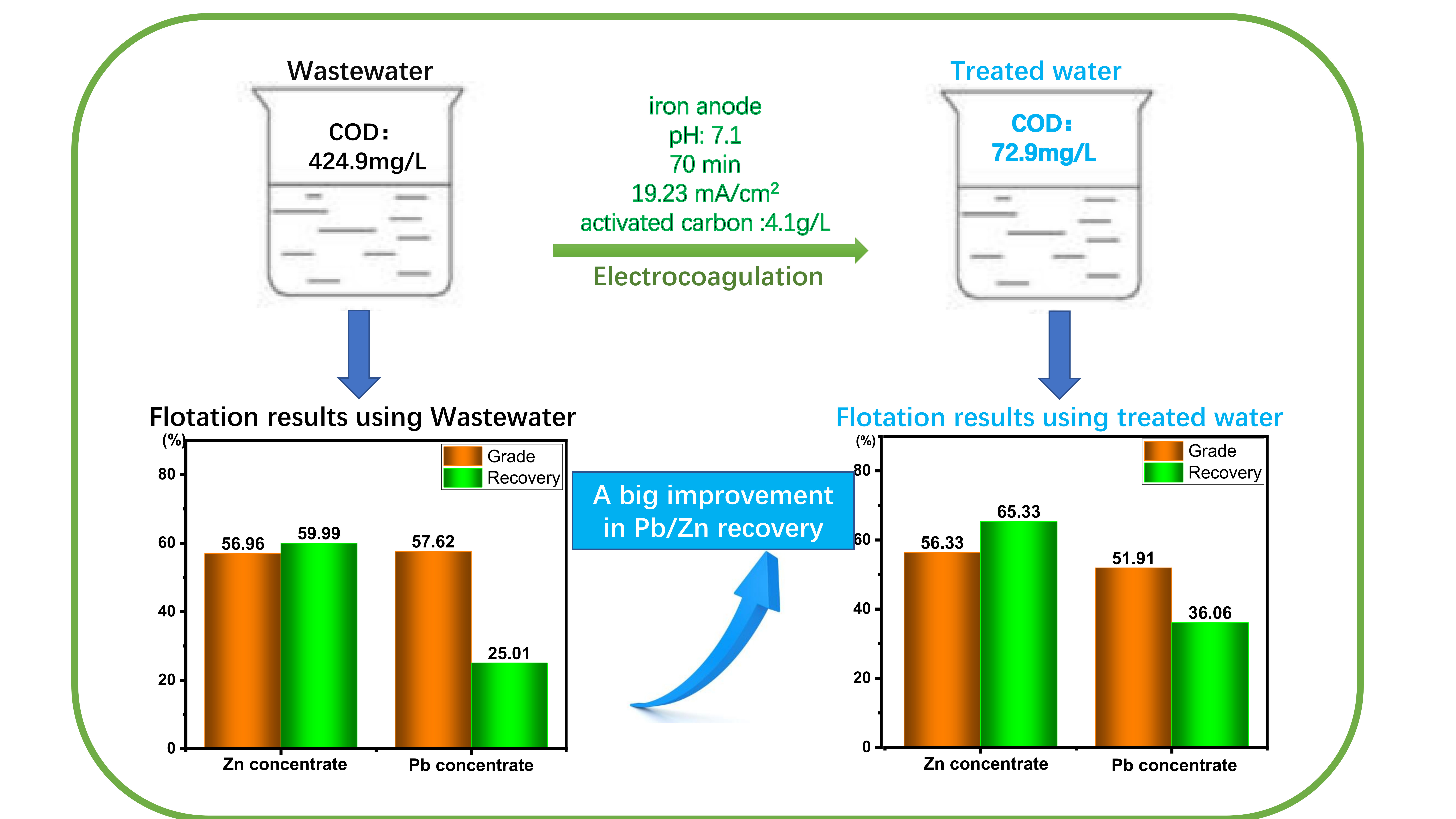 This picture shows Electrocoagulation review.
This picture shows Electrocoagulation review.
Design of electrocoagulation unit
 This image shows Design of electrocoagulation unit.
This image shows Design of electrocoagulation unit.
Electrocoagulation process
 This picture illustrates Electrocoagulation process.
This picture illustrates Electrocoagulation process.
Electrocoagulation wastewater treatment pdf
 This picture illustrates Electrocoagulation wastewater treatment pdf.
This picture illustrates Electrocoagulation wastewater treatment pdf.
Application of electrocoagulation
 This image illustrates Application of electrocoagulation.
This image illustrates Application of electrocoagulation.
Electrocoagulation in the treatment of industrial waters and wastewaters
 This image representes Electrocoagulation in the treatment of industrial waters and wastewaters.
This image representes Electrocoagulation in the treatment of industrial waters and wastewaters.
How are metal cations produced in electrocoagulation cell?
With this technology, metal cations are produced on the electrodes via electrolysis and these cations form various hydroxides in the water depending on the water pH. In addition to this main reaction, several side reactions, such as hydrogen bubble formation and the reduction of metals on cathodes, also take place in the cell.
Where was the study of electrocoagulation carried out?
This study was carried out between 2006 and 2011 at the Laboratory of Green Chemistry, Mikkeli, Finland with financial support from the European Union, the City of Mikkeli, Savcor Forest Oy and UPM-Kymmene Oyj.
Which is a better electrode material for electrocoagulation?
According to results of this study, aluminium is more suitable electrode material for electrocoagulation applications because it produces Al(III) species. Metal ions and hydroxides produced by iron electrodes are less effective in the destabilisation of pollutants because iron electrodes produce more soluble and less charged Fe(II) species.
How is electrocoagulation used to treat raw water?
Electrocoagulation technology has also been proposed for the treatment of raw waters and wastewaters. With this technology, metal cations are produced on the electrodes via electrolysis and these cations form various hydroxides in the water depending on the water pH.
Last Update: Oct 2021